Learn more about the advancements Novartis is making for patients living with cancer
Explore the science, production, and future of radioligand therapies (RLTs).
Leading the Science of RLT
Novartis pioneered radioligand therapy and developed the first FDA-approved RLT1
At Novartis, we believe that RLTs will transform oncology treatment and bring new options to people living with cancer.
RLT is in a class by itself
RLT evolved from 2 critical building blocks in oncology: the controlled use of radiation therapy and the emergence of targeted medicine.2
RLT was built on previous scientific breakthroughs1-3
RLT was built on previous scientific breakthroughs1-3
Reimagining the future of RLT
With the goal of improving and extending lives, Novartis is investigating a broad number of RLTs to reimagine the pillars of oncology treatment.
There are currently hundreds of unique RLT clinical and preclinical projects in development across a broad range of potential indications industry-wide.4
Our ambition is to reach every patient who could benefit from RLTs by expanding into more tumor types and moving RLTs into earlier lines of treatment5
Learn more about the next essential pillar of oncology
.
At Novartis, we are making the promise of next-generation medicines a reality
Learn about our pipeline
Interested in learning more?
The RLT Experience
Novartis is committed to providing RLT treatment to more people living with cancer and leveraging our expertise to deliver an exceptional RLT experience.
>37,000 patients to date have been treated with RLTs from Novartis6,7
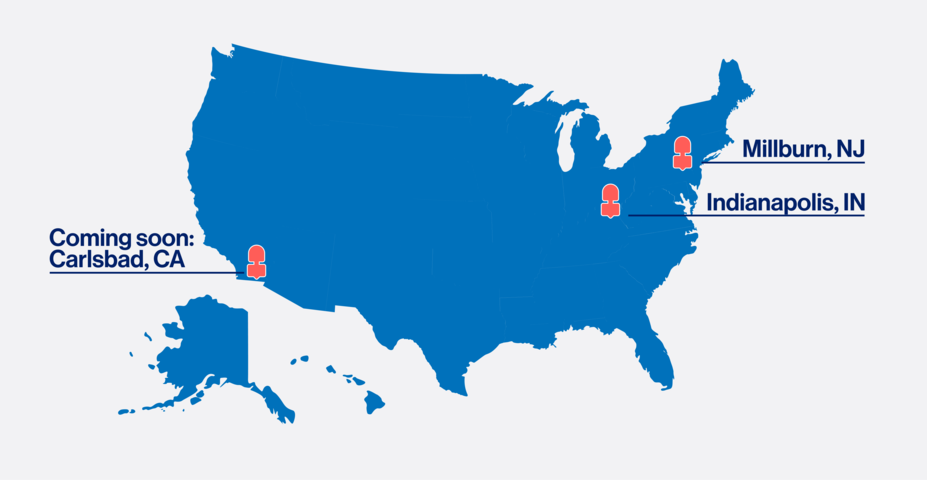
Manufacturing
The number of Novartis RLT manufacturing sites is planned to triple in the US and abroad. We continue to invest in developing a robust infrastructure with the ability to increase production to meet future demand.
RLT manufacturing requires highly specialized associates racing against the clock every day as they seek to advance the Novartis mission of reimagining medicine for patients.8
See the state-of-the-art process that brings RLTs to the patients who need them
.
Production
Novartis had an RLT production capacity of 250,000 doses in 2024. We have continued to expand our worldwide RLT manufacturing network, and with unconstrained supply, we’ve increased production for accelerated delivery of RLTs to meet current and future demand.5,9
Delivery
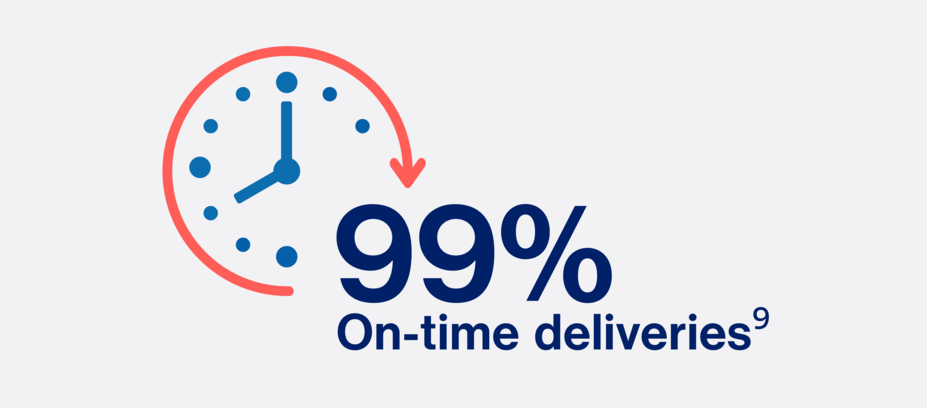
With a 99% on-time delivery rate, Novartis is making sure our RLT treatments get to patients as quickly as possible.9
Every RLT dose is made specifically for each patient. Novartis RLTs can be delivered to most treatment sites within 5 days of order placement, allowing your patients to begin treatment as soon as possible.10
Cancer moves fast. We move faster.
Novartis Patient Support™
Novartis Patient Support provides comprehensive resources designed to help your patients start, stay, and save on RLT.
The Promise of RLT
We see the limits of what's possible as a mere suggestion
Radioligand therapy is more than an advancement. It's a turning point
We are committed to bringing RLTs to more people living with cancer
|
Our RLT products
Learn more about integrating RLTs into your practice
Contact a Rep
Request more info
Keep up to date on the latest news and developments in RLT from Novartis.